What is in a Name? / Cosa c'è in un nome?

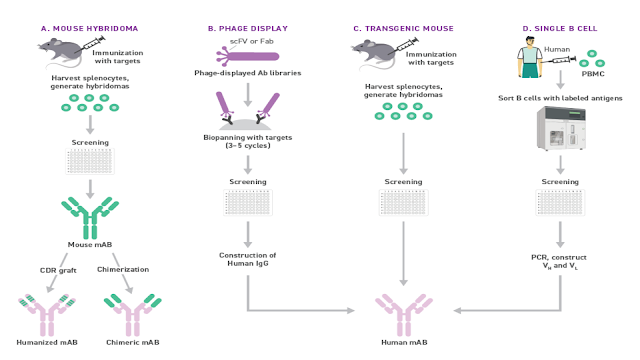
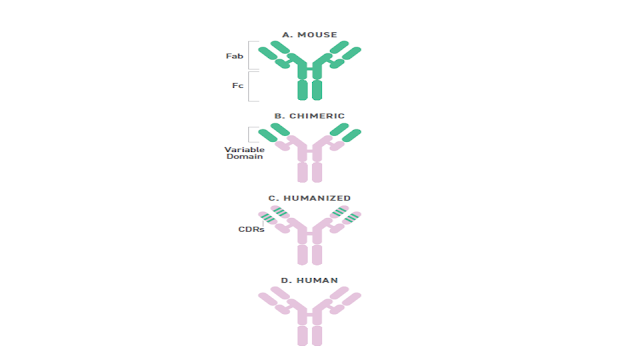
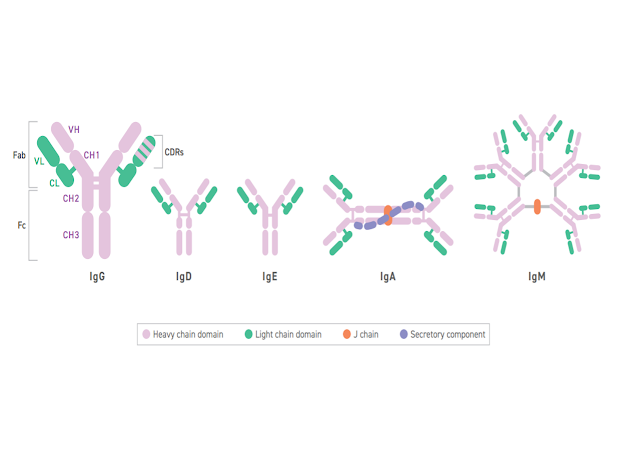
What is in a Name? / Cosa c'è in un nome? Segnalato dal Dott. Giuseppe Cotellessa / Reported by Dr. Giuseppe Cotellessa Adalimumab, raxibacumab, and belimumab: There’s a method to this naming madness. The International Nonproprietary Name (INN)—an expert group within the World Health Organization (WHO)—oversees the official naming formula, which calls out specific mAb features including the intended target, original host, modifications, and conjugations. Monoclonal antibodies named prior to 2017 included two other sections. A second substem or infix was used to denote the source or host organism that the antibody was originally produced in (most commonly “u” for human or “o” for mouse). An additional modification was also used to indicate chimeric (“-xi-”) or humanized (“-zu-”) engineering. Due in part to ambiguities and concerns that the names were being manipulated for commercial reasons, these two features were scrapped when the new INN guidelines were introduced. ITALIANO Ad