HTRF HMGB1, IL-1β AND IL-18 ASSAYS DISCRIMINATE BETWEEN INFLAMMASOME TRIGGERED PYROPTOSIS, AND NECROPTOSIS / I SAGGI HTRF HMGB1, IL-1β E IL-18 DISCRIMINANO TRA INFIAMMASONE TRIGGERATA PIROPOPTOSI E NECROPTOSI
HTRF HMGB1, IL-1β AND IL-18 ASSAYS
DISCRIMINATE BETWEEN INFLAMMASOME TRIGGERED PYROPTOSIS, AND NECROPTOSIS / I SAGGI HTRF HMGB1, IL-1β E IL-18 DISCRIMINANO TRA INFIAMMASONE TRIGGERATA PIROPOPTOSI E NECROPTOSI
Segnalato dal Dott. Giuseppe Cotellessa / Reported by Dr. Giuseppe Cotellessa
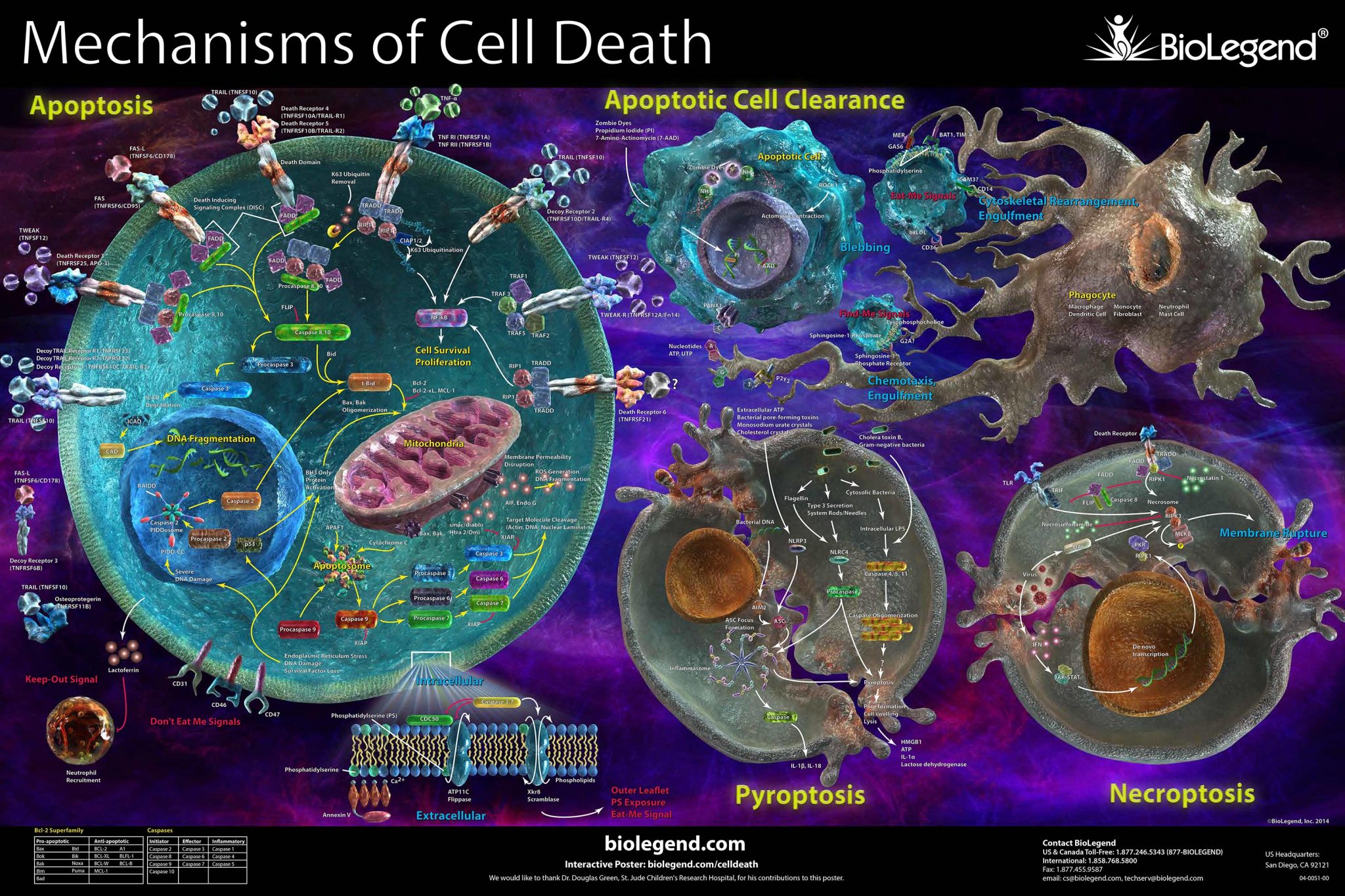
Two phenomena discovered very recently complete the panorama of cell death, solving the borderline cases between necrosis and pyroptosis. Inflammatory responses are characterized by the release of endogenous DAMPs also known as alarmins. During non-apoptotic programmed or regulated cell death, such as pyroptosis and necroptosis, HMGB1 is released along with other pro-inflammatory molecules. Pyroptosis relies on two steps. The first step is called “priming”. Following TLR stimulation (e.g LPS), the expression of NLRP3 and pro-IL-1β is upregulated through NF-kB transcription factor. In the second “activation” step, DAMPs such as extracellular ATP, or the bacterial toxin nigericin, are sensed by NLRP3 which oligomerizes, leading to the activation of the NLRP3 inflammasome. In turn, it induces the cleavage of pro-caspase-1 to its active form. Caspase-1 then cleaves the pro-forms of the inflammatory IL-1β and IL-18, that are released as their mature forms. Parallelly caspase-1 induces the cleavage of proGSDMD which forms pores at the cell membrane and the subsequent release of IL-1β, IL-18 and HMGB1. Therefore pyroptosis is characterized by the simultaneous secretion of HMGB1 together with mature IL-1β and IL-18. Necroptosis involves the loss of membrane integrity and release of DAMPs. Necroptosis involves RIPK1 and/or RIPK3 and proceeds through a caspase-independent pathway (2,3). Necroptosis is promoted by death receptor activation. Upon TNFα binding, the cytosolic death domain of TNFR1 recruits a prosurvival complex, consisting of TRADD, RIPK1 and cIAP. This complex can either initiates apoptosis through the activation of caspase-8 or initiate necroptosis when cIAP and caspase-8 are absent or inactive. In this case RIPK1 associates with oligomerized RIPK3 and MLKL to form the necrosome. RIPK3 phosphorylates MLKL which forms plasma membrane pores and triggers the release of HMGB1. Contrary to pyroptosis, necroptosis is associated with HMGB1 release but not mature IL-1β/IL-18. Inflammasome dysregulation has been associated with pathologies, such as metabolic, autoimmune diseases, neurodegenerative or cardiovascular diseases. Thus inflammasome components, like NLRP3, are attractive drug targets. Whereas NLRP3 agonists are investigated in immuno-oncology to enhance immune function, antagonists display broader applications for autoimmune and chronic inflammatory diseases, metabolic disorders and ageing related pathologies. In this note, we show that the release of HMGB1 induced in the context of pyroptosis and necroptosis can be easily monitored with the HTRF HMGB1 kit. In addition we provide data comparison with two other methods, luciferase and ELISA and establish well correlated pharmacological responses. Finally we demonstrate that the use of HTRF HMGB1 kit along with HTRF IL-1β and IL-18 represent a valuable approach to decipher between pyroptosis and necroptosis biological responses.
The HMGB1 HTRF kit allows for the quantitation of the native HMGB1 released in pyroptosis and necroptosis and can distinguish between the two when used in combination with HTRF IL-1β/IL-18 kits. Contrary to luciferase assay, HTRF HMGB1 assay can be applied to unmodified cells, being more universal. Unlike the ELISA, this HTRF HMGB1 assay is insensitive to the bovine HMGB1 present in the FCS. Besides no wash steps required, the wider dynamic range of the HTRF kit makes it easier to use since no extra dilution of the supernatants were required, at least in this study.
ITALIANO
Segnalato dal Dott. Giuseppe Cotellessa / Reported by Dr. Giuseppe Cotellessa

Two phenomena discovered very recently complete the panorama of cell death, solving the borderline cases between necrosis and pyroptosis. Inflammatory responses are characterized by the release of endogenous DAMPs also known as alarmins. During non-apoptotic programmed or regulated cell death, such as pyroptosis and necroptosis, HMGB1 is released along with other pro-inflammatory molecules. Pyroptosis relies on two steps. The first step is called “priming”. Following TLR stimulation (e.g LPS), the expression of NLRP3 and pro-IL-1β is upregulated through NF-kB transcription factor. In the second “activation” step, DAMPs such as extracellular ATP, or the bacterial toxin nigericin, are sensed by NLRP3 which oligomerizes, leading to the activation of the NLRP3 inflammasome. In turn, it induces the cleavage of pro-caspase-1 to its active form. Caspase-1 then cleaves the pro-forms of the inflammatory IL-1β and IL-18, that are released as their mature forms. Parallelly caspase-1 induces the cleavage of proGSDMD which forms pores at the cell membrane and the subsequent release of IL-1β, IL-18 and HMGB1. Therefore pyroptosis is characterized by the simultaneous secretion of HMGB1 together with mature IL-1β and IL-18. Necroptosis involves the loss of membrane integrity and release of DAMPs. Necroptosis involves RIPK1 and/or RIPK3 and proceeds through a caspase-independent pathway (2,3). Necroptosis is promoted by death receptor activation. Upon TNFα binding, the cytosolic death domain of TNFR1 recruits a prosurvival complex, consisting of TRADD, RIPK1 and cIAP. This complex can either initiates apoptosis through the activation of caspase-8 or initiate necroptosis when cIAP and caspase-8 are absent or inactive. In this case RIPK1 associates with oligomerized RIPK3 and MLKL to form the necrosome. RIPK3 phosphorylates MLKL which forms plasma membrane pores and triggers the release of HMGB1. Contrary to pyroptosis, necroptosis is associated with HMGB1 release but not mature IL-1β/IL-18. Inflammasome dysregulation has been associated with pathologies, such as metabolic, autoimmune diseases, neurodegenerative or cardiovascular diseases. Thus inflammasome components, like NLRP3, are attractive drug targets. Whereas NLRP3 agonists are investigated in immuno-oncology to enhance immune function, antagonists display broader applications for autoimmune and chronic inflammatory diseases, metabolic disorders and ageing related pathologies. In this note, we show that the release of HMGB1 induced in the context of pyroptosis and necroptosis can be easily monitored with the HTRF HMGB1 kit. In addition we provide data comparison with two other methods, luciferase and ELISA and establish well correlated pharmacological responses. Finally we demonstrate that the use of HTRF HMGB1 kit along with HTRF IL-1β and IL-18 represent a valuable approach to decipher between pyroptosis and necroptosis biological responses.
The HMGB1 HTRF kit allows for the quantitation of the native HMGB1 released in pyroptosis and necroptosis and can distinguish between the two when used in combination with HTRF IL-1β/IL-18 kits. Contrary to luciferase assay, HTRF HMGB1 assay can be applied to unmodified cells, being more universal. Unlike the ELISA, this HTRF HMGB1 assay is insensitive to the bovine HMGB1 present in the FCS. Besides no wash steps required, the wider dynamic range of the HTRF kit makes it easier to use since no extra dilution of the supernatants were required, at least in this study.
ITALIANO
Due fenomeni scoperti molto di recente completano il
panorama della morte cellulare, risolvendo i casi limite tra piroptosi ed
apoptosi.
Le risposte infiammatorie sono caratterizzate dal rilascio di DAMP endogeni noti anche come allarmine. Durante la morte cellulare programmata o regolata non apoptotica, come la pirotosi e la necroptosi, l'HMGB1 viene rilasciato insieme ad altre molecole pro-infiammatorie. La piroptosi si basa su due passaggi. Il primo passo si chiama "priming". Dopo la stimolazione TLR (ad esempio LPS), l'espressione di NLRP3 e pro-IL-1β è sovraregolata attraverso il fattore di trascrizione NF-kB. Nella seconda fase di "attivazione", i DAMP come l'ATP extracellulare o la nigericina tossina batterica, vengono rilevati dall'NLRP3 che oligomerizza, portando all'attivazione dell'inflammasoma NLRP3. A sua volta, induce la scissione di pro-caspase-1 nella sua forma attiva. Caspase-1 quindi fende le pro-forme di IL-1β e IL-18 infiammatorie, che vengono rilasciate come loro forme mature. Parallelamente caspase-1 induce la scissione di proGSDMD che forma i pori sulla membrana cellulare e il successivo rilascio di IL-1β, IL-18 e HMGB1. Pertanto la pirotosi è caratterizzata dalla secrezione simultanea di HMGB1 insieme a IL-1β maturo e IL-18. La necroptosi comporta la perdita di integrità della membrana e il rilascio di DAMP. La necroptosi coinvolge RIPK1 e / o RIPK3 e procede attraverso un percorso indipendente dalla caspasi (2,3). La necroptosi è promossa dall'attivazione del recettore della morte. Su legame TNFα, il dominio della morte citosolica di TNFR1 recluta un complesso prosurvival, costituito da TRADD, RIPK1 e cIAP. Questo complesso può iniziare l'apoptosi attraverso l'attivazione di caspase-8 o iniziare la necroptosi quando cIAP e caspase-8 sono assenti o inattivi. In questo caso RIPK1 si associa a RIPK3 oligomerizzato e MLKL per formare il necrosoma. RIPK3 fosforilati MLKL che forma i pori della membrana plasmatica e innesca il rilascio di HMGB1. Contrariamente alla pirotosi, la necroptosi è associata al rilascio di HMGB1 ma non a IL-1β / IL-18 maturo. La disregolazione dell'inflammasoma è stata associata a patologie come malattie metaboliche, autoimmuni, malattie neurodegenerative o cardiovascolari. Pertanto i componenti dell'inflammasoma, come NLRP3, sono bersagli farmacologici interessanti. Mentre gli agonisti della NLRP3 sono studiati in immuno-oncologia per migliorare la funzione immunitaria, gli antagonisti mostrano applicazioni più ampie per malattie infiammatorie autoimmuni e croniche, disturbi metabolici e patologie correlate all'invecchiamento. In questa nota, mostriamo che il rilascio di HMGB1 indotto nel contesto di pirotosi e necroptosi può essere facilmente monitorato con il kit HTRF HMGB1. Inoltre forniamo il confronto dei dati con altri due metodi, la luciferasi e l'ELISA e stabiliamo risposte farmacologiche ben correlate. Infine, dimostriamo che l'uso del kit HTRF HMGB1 insieme a HTRF IL-1β e IL-18 rappresentano un valido approccio per la decifrazione tra le risposte biologiche di piroptosi e necroptosi.
Il kit HMGB1 HTRF consente la quantificazione dell'HMGB1 nativo rilasciato in piroptosi e necroptosi e può distinguere tra i due quando utilizzato in combinazione con i kit HTRF IL-1β / IL-18. Contrariamente al saggio della luciferasi, il saggio HTRF HMGB1 può essere applicato a cellule non modificate, essendo più universale. A differenza dell'ELISA, questo test HTRF HMGB1 è insensibile al bovino HMGB1 presente nell'FCS. Oltre alle fasi di lavaggio non necessarie, la gamma dinamica più ampia del kit HTRF ne semplifica l'utilizzo poiché non è richiesta alcuna diluizione aggiuntiva dei surnatanti, almeno in questo studio.
Da:



Commenti
Posta un commento